Mycoplasma hominis bloodstream infection and persistent pneumonia in a neurosurgery patient: a case report - BMC Infectious Diseases - BMC Infectious Diseases
July 2019, a 56-year-old male was admitted to the Emergency Department of the Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China, after suffering from unconsciousness for five hours. The patient displayed inability to stand, limb twitching, nausea and vomiting, and he did not have an obvious inducement for sudden unconsciousness. Physical examination upon admission showed that he was able to open his eyes upon pain stimulation, and he furthermore showed aphasia, bilateral pupils with a diameter of 3 mm, sensitivity to light, right limb muscle strength Grade II, bilateral breathing sounds and rales, and right Pap sign (+) (indicating left cerebral infarction). A cerebral computed tomography (CT) scan identified a left basal ganglia hematoma and right basal calcification (Fig. 1a). Furthermore, the patient showed pulmonary emphysema with inflammation of the lower lobe of the left lung and a small amount of pleural effusion on both sides. The patient's family reported that before this episode the patient was in good health and did not suffer from diabetes or any immune diseases and that he was not on any medications. Furthermore, no problems were detected by transesophageal echocardiography and the patient did not display any symptoms of endocarditis, indicating that a mycotic aneurisms was unlikely. After emergency treatment to control blood pressure, lowering intracranial pressure and hemostasis, re-examination by cranial CT scan showed that bleeding had progressed. The patient was then referred to the Department of Neurosurgery and on the same day he underwent microsurgery and decompressive craniectomy. Approximately 30 ml of the hematoma was removed during the surgery, and a drainage tube was placed in the hematoma cavity after hemostasis. The patient was returned to the ward with a body temperature of 36.8 °C and tracheal intubation because of preoperative vomiting and lung inflammation and he was treated empirically with piperacillin-tazobactam (4.5 g IV q8h). The drainage tube from the hematoma cavity was removed on the 3rd day, while a pre-operatively placed catheter was removed on the 7th day after admission and both remained negative in subsequent culture tests.
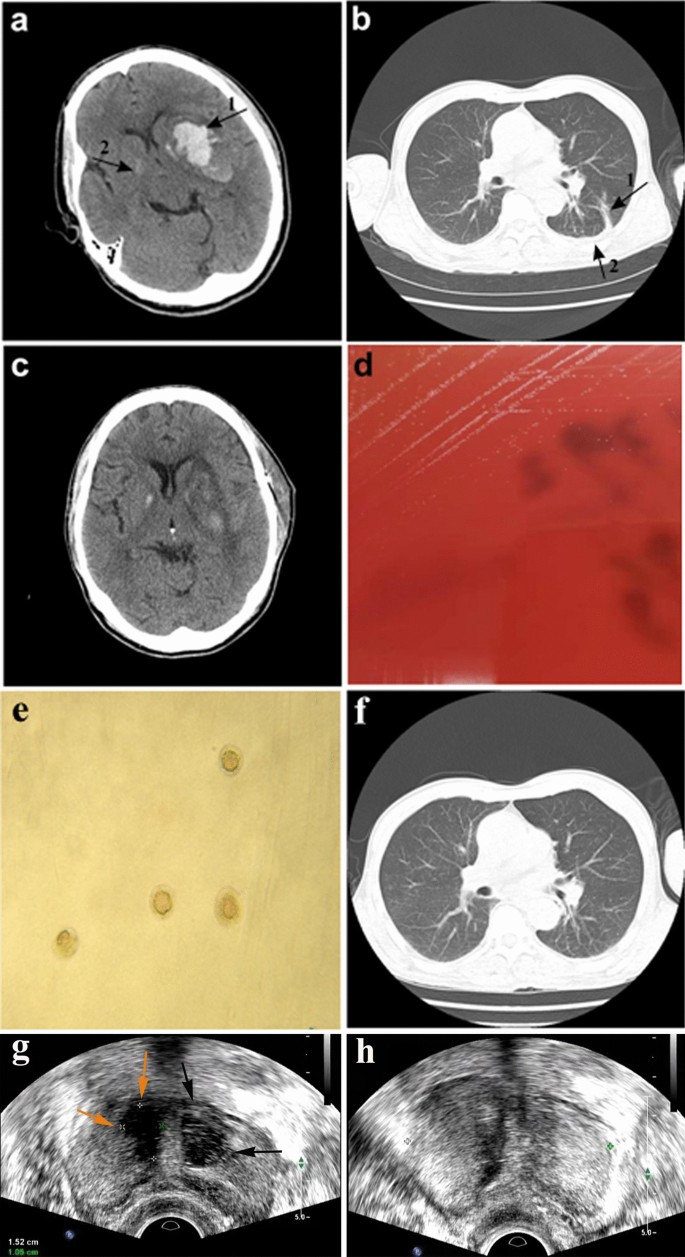
Computed tomography (CT) images of the brain and chest before and after microsurgery or antimicrobial treatment. a Brain CT scan of the patient showing cerebral hemorrhage on day 1 of hospital admission. Arrow 1: left basal ganglia hematoma. Arrow 2: right basal calcification. b Chest CT scan of the patient on day 8 after hospital admission showing fluid accumulation in the lungs. Arrow 1: inflammatory lesions in the lower lobe of the left lung. Arrow 2: small amount of pleural effusion on both sides of the chest. c CT scan of the brain on day 16 after hospital admission showing good recovery after surgery. d Development of pin-point-sized colonies after 48 h sub-culturing of the left side anaerobic culture bottle on Columbia blood agar at 37 °C in the presence of 5% CO2. e Development of typical M. hominis fried-egg-shaped colonies after sub-culturing on A7 agar. f Chest CT scan of the patient on day 33 after hospital admission showing absorption of chest effusion. g Transrectal ultrasound (TRUS) scan of the prostate at 1-month follow-up showing right-side (yellow arrow) and left-side (black arrows) prostate abscesses. h TRUS scan of the prostate 15 days after the drainage procedure showing return to a healthy state
On the 8th day after hospital admission, the patient had a sudden fever with a body temperature of 38.6 °C and a lung CT scan showed inflammatory lesions in the lower lobe of the left lung, and a small amount of pleural effusion on both sides of the chest (Fig. 1b). Due to empirical therapy considerations for a likely Gram-negative bacterial infection, piperacillin-tazobactam was discontinued and meropenem therapy (0.5 g IV q8h) was initiated. The patient's pre-operatively placed urinary catheter was removed on the 15th day after hospital admission and both the catheter and urine samples remained culture negative, while urine routine was normal. However, the patient's body temperature remained elevated and on the 16th day after hospital admission, the patient started to cough-up yellow sticky sputum. However, he did not display chest tightness or shortness of breath and his right limb hemiplegia and scalp incision healed well (Fig. 1c). On the 18th day after admission, his white blood cell (WBC) counts were 9.6 × 109/L with a neutrophil % of 86.5% and C-reactive protein (CRP) levels were 17.2 mg/L. Therefore, bilateral blood samples were drawn for aerobic (BacT/ALERT FA, bioMérieux) and anaerobic (BacT/ALERT SN, bioMérieux) culturing to investigate a possible bacterial infection. In addition, antimicrobial treatment was modified to meropenem (0.5 g IV q8h) in combination with ceftriaxone (2 g IV qd). On the 21st day after admission, one of the anaerobic culture bottle was positive for growth, but no bacteria were identified by Gram-staining. Subsequent sub-culturing resulted in pin-point-sized colonies on Columbia blood agar plates (bioMérieux) by day 23 after admission (Fig. 1d), which were identified as M. hominis by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; VITEK MS, bioMérieux) and further confirmed by16S rRNA sequencing and growth as typical fried-egg-shaped colonies on A7 agar (Fig. 1e). In the meantime, treatment was modified on the 22nd day after admission to linezolid (600 mg IV q12h) combined with meropenem (0.5 g IV q8h) and discontinued for ceftriaxone. Furthermore, a lumbar puncture on the 22nd day did not display any evidence of cerebrospinal fluid (CSF) infection by routine laboratory tests. A BAL was also performed on the 22nd day given the persistence of pulmonary symptoms. BAL fluid was used for next-generation sequencing (BGI-Nanjing) and bioinformatics analysis as described previously [3]. Shortly, DNA was extracted using the TIANamp Micro DNA Kit (DP316, TIANGEN BIOTECH) and generated DNA Nanoballs (DNB) were sequenced by the MGISEQ-2000 platform, followed by pathogen identification after alignment with genome sequences obtained from the Pathogens Metagenomics Database (PMDB). Sequencing results on the 24th day identified M. hominis as the main pathogenic bacterium in the BAL fluid (Table 1). After identification of M. hominis on day 23 after hospital admission, treatment was modified to ciprofloxacin (400 mg IV q12). However, by day 25 after admission antimicrobial susceptibility testing according to the broth microdilution method [4] showed that the M. hominis strain was resistant to ciprofloxacin and susceptible to doxycycline and moxifloxacin. Therefore, treatment was modified to doxycycline (100 mg po bid) combined with moxifloxacin (400 mg IV qd). The following 8 days, the patient's fever gradually declined and his lung inflammation improved with clear absorption of chest effusion (Fig. 1f). On the 33th day after admission, based on requests by the patient's family the patient was transferred to a hospital in the patient's hometown and continued for another 7 days on doxycycline (100 mg po bid) combined with moxifloxacin (400 mg IV qd).
At 1-month follow-up, cerebral CT examination displayed no abnormalities, while chest CT revealed showed obvious absorption of the pulmonary inflammation. Furthermore, the patient's body temperature and WBC counts were normal. However, the patient complained of dysuria and perineal pain and he was therefore referred to the urologist. Rectal examination revealed an enlarged, firm prostate with a middle lateral nodule, but without signs of fluctuations. But urine cultures remained negative. The patient was then referred for transrectal ultrasound (TRUS) with a pre-diagnosis of prostate abscess. TRUS showed and enlarged prostate (50 × 50 × 41 mm) and multiple lesions in the transition zone of the prostate. These thick-walled hypo-echoic lesions with internal echogenicities resembled abscesses. Both the right-side lesion (15 × 11 mm) and the left side lesion (18 × 12 mm) were uniloculated (Fig. 1g). The patient was directly hospitalized and started on intravenous doxycycline (100 mg IV q12), followed 2 days later by ultrasound-guided transperineal drainage of the abscesses and flushing with doxycycline. The aspirate subsequently cultured M. hominis, but whole-genome sequencing for comparison with the earlier isolate was not performed. A 24 h follow-up TRUS control was abscess negative, but hypoechoic areas that seemed like inflammatory changes were seen in the abscess location. The patient was discharged from the hospital, as his clinical symptoms were resolved. The patient was maintained on oral doxycycline (100 mg po bid) for 14 days after the intervention. On the follow-up TRUS control 15 days after the procedure, abscess recurrence was not seen (Fig. 1h), while at the 1-month follow-up TRUS lesions had disappeared and the prostate gland was no longer enlarged. Cerebral and chest CT examinations were performed at 1-year follow-up and displayed no abnormalities.
Comments
Post a Comment