Secreted fungal virulence effector triggers allergic inflammation via TLR4 - Nature.com
Abstract
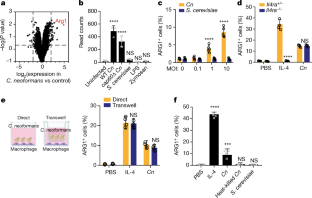
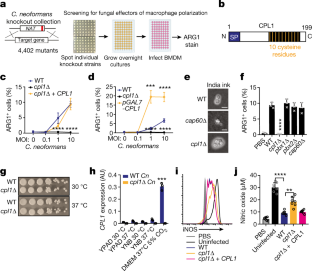
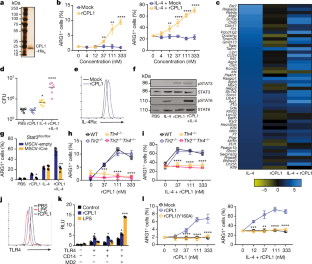
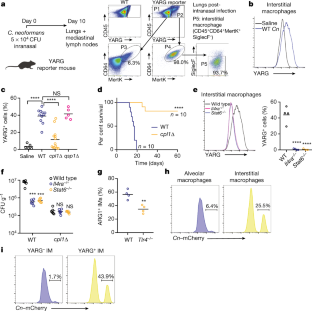
Invasive fungal pathogens are major causes of human mortality and morbidity1,2. Although numerous secreted effector proteins that reprogram innate immunity to promote virulence have been identified in pathogenic bacteria, so far, there are no examples of analogous secreted effector proteins produced by human fungal pathogens. Cryptococcus neoformans, the most common cause of fungal meningitis and a major pathogen in AIDS, induces a pathogenic type 2 response characterized by pulmonary eosinophilia and alternatively activated macrophages3,4,5,6,7,8. Here, we identify CPL1 as an effector protein secreted by C. neoformans that drives alternative activation (also known as M2 polarization) of macrophages to enable pulmonary infection in mice. We observed that CPL1-enhanced macrophage polarization requires Toll-like receptor 4, which is best known as a receptor for bacterial endotoxin but is also a poorly understood mediator of allergen-induced type 2 responses9,10,11,12. We show that this effect is caused by CPL1 itself and not by contaminating lipopolysaccharide. CPL1 is essential for virulence, drives polarization of interstitial macrophages in vivo, and requires type 2 cytokine signalling for its effect on infectivity. Notably, C. neoformans associates selectively with polarized interstitial macrophages during infection, suggesting a mechanism by which C. neoformans generates its own intracellular replication niche within the host. This work identifies a circuit whereby a secreted effector protein produced by a human fungal pathogen reprograms innate immunity, revealing an unexpected role for Toll-like receptor 4 in promoting the pathogenesis of infectious disease.
This is a preview of subscription content
Access options
Subscribe to Journal
Get full journal access for 1 year
199,00 €
only 3,90 € per issue
Tax calculation will be finalised during checkout.
Buy article
Get time limited or full article access on ReadCube.
$32.00
All prices are NET prices.
Data availability
The primary read files as well as expression count files for RNA-seq data in this paper are available to download from the Gene Expression Omnibus under accession number GSE203483. Source data are provided with this paper.
References
Armstrong-James, D., Meintjes, G. & Brown, G. D. A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol. 22, 120–127 (2014).
Brown, G. D. et al. Hidden killers: human fungal infections. Sci. Transl. Med. 4, 165rv13 (2012).
Zhao, Y., Lin, J., Fan, Y. & Lin, X. Life cycle of Cryptococcus neoformans. Annu. Rev. Microbiol. 73, 17–42 (2019).
Müller, U. et al. Abrogation of IL-4 receptor-α-dependent alternatively activated macrophages is sufficient to confer resistance against pulmonary cryptococcosis despite an ongoing Th2 response. Int. Immunol. 25, 459–470 (2013).
Mueller, U. et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J. Immunol. 179, 5367–5377 (2007).
Wiesner, D. L. et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 11, e1004701 (2015).
Schulze, B. et al. CD4+FoxP3+ regulatory T cells suppress fatal T helper 2 cell immunity during pulmonary fungal infection. Eur. J. Immunol. 44, 3596–3604 (2014).
Stenzel, W. et al. IL-4/IL-13-dependent alternative activation of macrophages but not microglial cells is associated with uncontrolled cerebral cryptococcosis. Am. J. Pathol. 174, 486–496 (2009).
Trompette, A. et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457, 585–588 (2009).
Hammad, H. et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 15, 410–416 (2009).
Eisenbarth, S. C. et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196, 1645–1651 (2002).
Millien, V. O. et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 341, 792–796 (2013).
Ademe, M. & Girma, F. Candida auris: from multidrug resistance to pan-resistant strains. Infect. Drug Resist. 13, 1287–1294 (2020).
Wall, G. & Lopez-Ribot, J. L. Current antimycotics, new prospects, and future approaches to antifungal therapy. Antibiotics 9, 445 (2020).
Selin, C., de Kievit, T. R., Belmonte, M. F. & Fernando, W. G. D. Elucidating the role of effectors in plant-fungal interactions: progress and challenges. Front. Microbiol. 7, 600 (2016).
Rajasingham, R. et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 17, 873–881 (2017).
Mueller, U. et al. Lack of IL-4 receptor expression on T helper cells reduces T helper 2 cell polyfunctionality and confers resistance in allergic bronchopulmonary mycosis. Mucosal Immunol. 5, 299–310 (2012).
Kindermann, M. et al. Group 2 innate lymphoid cells (ILC2) suppress beneficial type 1 immune responses during pulmonary cryptococcosis. Front. Immunol. 11, 209 (2020).
May, R. C., Stone, N. R. H., Wiesner, D. L., Bicanic, T. & Nielsen, K. Cryptococcus: from environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 14, 106–117 (2016).
Vecchiarelli, A. Immunoregulation by capsular components of Cryptococcus neoformans. Med. Mycol. 38, 407–417 (2000).
Liu, O. W. et al. Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell 135, 174–188 (2008).
Homer, C. M. et al. Intracellular action of a secreted peptide required for fungal virulence. Cell Host Microbe 19, 849–864 (2016).
Stergiopoulos, I. & de Wit, P. J. G. M. Fungal effector proteins. Annu. Rev. Phytopathol. 47, 233–263 (2009).
Arras, S. D. M., Chitty, J. L., Blake, K. L., Schulz, B. L. & Fraser, J. A. A genomic safe haven for mutant complementation in Cryptococcus neoformans. PLoS ONE 10, e0122916 (2015).
Brown, J. C. S. et al. Unraveling the biology of a fungal meningitis pathogen using chemical genetics. Cell 159, 1168–1187 (2014).
Kumar, P. et al. Pbx proteins in Cryptococcus neoformans cell wall remodeling and capsule assembly. Eukaryot. Cell 13, 560–571 (2014).
Kawakami, K., Zhang, T., Qureshi, M. H. & Saito, A. Cryptococcus neoformans inhibits nitric oxide production by murine peritoneal macrophages stimulated with interferon-gamma and lipopolysaccharide. Cell. Immunol. 180, 47–54 (1997).
Gibbs, K. D. et al. The Salmonella secreted effector SarA/SteE mimics cytokine receptor signaling to activate STAT3. Cell Host Microbe 27, 129–139.e4 (2020).
Panagi, I. et al. Salmonella effector SteE converts the mammalian serine/threonine kinase GSK3 into a tyrosine kinase to direct macrophage polarization. Cell Host Microbe 27, 41–53.e6 (2020).
Kasmi, El,K. C. et al. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat. Immunol. 9, 1399–1406 (2008).
Deguine, J. & Barton, G. M. MyD88: a central player in innate immune signaling. F1000Prime Rep. 6, 97 (2014).
Lind, N. A., Rael, V., Pestal, K., Liu, B. & Barton, G. M. Regulation of the nucleic acid-sensing Toll-like receptors. Nat. Rev. Immunol. 22, 224–235 (2022).
Lancaster, G. I. et al. Evidence that TLR4 is not a receptor for saturated fatty acids but mediates lipid-induced inflammation by reprogramming macrophage metabolism. Cell Metab. 27, 1096–1110.e5 (2018).
Zanoni, I. et al. CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147, 868–880 (2011).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Chevigné, A. & Jacquet, A. Emerging roles of the protease allergen Derp1 in house dust mite-induced airway inflammation. J. Allergy Clin. Immunol. 142, 398–400 (2018).
Jacquet, A. Characterization of innate immune responses to house dust mite allergens: pitfalls and limitations. Front. Allergy 2, 662378 (2021).
Evren, E., Ringqvist, E. & Willinger, T. Origin and ontogeny of lung macrophages: from mice to humans. Immunology 160, 126–138 (2020).
Makita, N., Hizukuri, Y., Yamashiro, K., Murakawa, M. & Hayashi, Y. IL-10 enhances the phenotype of M2 macrophages induced by IL-4 and confers the ability to increase eosinophil migration. Int. Immunol. 27, 131–141 (2015).
Price, J. V. & Vance, R. E. The macrophage paradox. Immunity 41, 685–693 (2014).
Chun, C. D. & Madhani, H. D. Applying genetics and molecular biology to the study of the human pathogen Cryptococcus neoformans. Methods Enzymol. 470, 797–831 (2010).
Acknowledgements
We thank G. Barton for provision of Tlr2−/−, Tlr4−/− and Tlr2−/−Tlr4−/− mice, and R. Ricardo-Gonzalez for provision of Il4ra−/− and Stat6−/− mice; S. Chou for advice on protein purification; J. Cyster and E. Goldberg for critically reading the manuscript, discussions and advice; and S. Catania and M. Boucher for discussions and advice. Support was provided by the Chan–Zuckerberg Biohub, US National Institutes of Health, Jane Coffin Childs Memorial Fund for Medical Research Fellowship and Beckman Foundation.