Impact of enteric bacterial infections at and beyond the epithelial barrier - Nature.com
Abstract
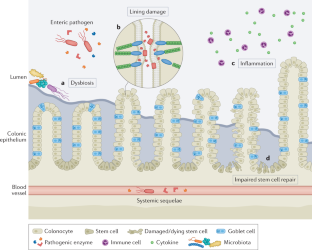
The mucosal lining of the gut has co-evolved with a diverse microbiota over millions of years, leading to the development of specialized mechanisms to actively limit the invasion of pathogens. However, some enteric microorganisms have adapted against these measures, developing ways to hijack or overcome epithelial micro-integrity mechanisms. This breach of the gut barrier not only enables the leakage of host factors out of circulation but can also initiate a cascade of detrimental systemic events as microbiota, pathogens and their affiliated secretions passively leak into extra-intestinal sites. Under normal circumstances, gut damage is rapidly repaired by intestinal stem cells. However, with substantial and deep perturbation to the gut lining and the systemic dissemination of gut contents, we now know that some enteric infections can cause the impairment of host regenerative processes. Although these local and systemic aspects of enteric disease are often studied in isolation, they heavily impact one another. In this Review, by examining the journey of enteric infections from initial establishment to systemic sequelae and how, or if, the host can successfully repair damage, we will tie together these complex interactions to provide a holistic overview of the impact of enteric infections at and beyond the epithelial barrier.
This is a preview of subscription content, access via your institution
Access options
Subscribe to Journal
Get full journal access for 1 year
99,00 €
only 8,25 € per issue
Tax calculation will be finalised during checkout.
Buy article
Get time limited or full article access on ReadCube.
$32.00
All prices are NET prices.
References
Karmakar, S., Deng, L., He, X. C. & Li, L. Intestinal epithelial regeneration: active versus reserve stem cells and plasticity mechanisms. Am. J. Physiol. Gastrointest. Liver Physiol. 318, G796–G802 (2020).
Barker, N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 15, 19–33 (2014).
Peck, B. C. E., Shanahan, M. T., Singh, A. P. & Sethupathy, P. Gut microbial influences on the mammalian intestinal stem cell niche. Stem Cell Int. 2017, 5604727 (2017).
Leslie, J. L. et al. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect. Immun. 83, 138–145 (2015).
Bonfini, A., Liu, X. & Buchon, N. From pathogens to microbiota: how Drosophila intestinal stem cells react to gut microbes. Dev. Comp. Immunol. 64, 22–38 (2016).
Hoffmann, W. Regeneration of the gastric mucosa and its glands from stem cells. Curr. Med. Chem. 15, 3133–3144 (2008).
Pitsouli, C., Apidianakis, Y. & Perrimon, N. Homeostasis in infected epithelia: stem cells take the lead. Cell Host Microbe 6, 301–307 (2009).
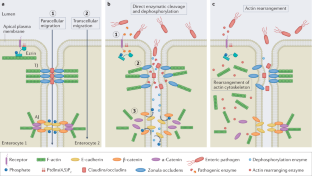
Backert, S., Boehm, M., Wessler, S. & Tegtmeyer, N. Transmigration route of Campylobacter jejuni across polarized intestinal epithelial cells: paracellular, transcellular or both? Cell Commun. Signal. 11, 72 (2013).
Buchon, N., Broderick, N. A., Chakrabarti, S. & Lemaitre, B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 23, 2333–2344 (2009).
McCormick, B. A., Stocker, B. A., Laux, D. C. & Cohen, P. S. Roles of motility, chemotaxis, and penetration through and growth in intestinal mucus in the ability of an avirulent strain of Salmonella Typhimurium to colonize the large intestine of streptomycin-treated mice. Infect. Immun. 56, 2209–2217 (1988).
Boyle, E. C., Brown, N. F. & Finlay, B. B. Salmonella enterica serovar Typhimurium effectors SopB, SopE, SopE2 and SipA disrupt tight junction structure and function. Cell Microbiol. 8, 1946–1957 (2006).
Prizont, R. & Reed, W. P. Differences in blood group B-specific mucinase activity between virulent and avirulent Shigella flexneri 2a strains. Microb. Pathog. 11, 129–135 (1991).
Silva, A. J., Pham, K. & Benitez, J. A. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 149, 1883–1891 (2003).
Hoy, B. et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 11, 798–804 (2010).
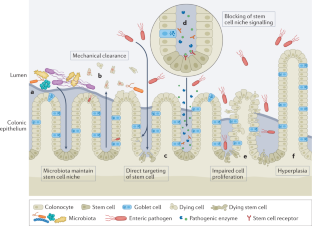
Mileto, S. J. et al. Clostridioides difficile infection damages colonic stem cells via TcdB, impairing epithelial repair and recovery from disease. Proc. Natl Acad. Sci. USA 117, 8064–8073 (2020). This is an important study demonstrating the direct interaction and impairment of colonic stem cells by a specific enteric pathogen.
Stanley, R. A., Ram, S. P., Wilkinson, R. K. & Roberton, A. M. Degradation of pig gastric and colonic mucins by bacteria isolated from the pig colon. Appl. Environ. Microbiol. 51, 1104–1119 (1986).
Lecuit, M. et al. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292, 1722–1725 (2001).
Boehm, M. et al. Rapid paracellular transmigration of Campylobacter jejuni across polarized epithelial cells without affecting TER: role of proteolytic-active HtrA cleaving E-cadherin but not fibronectin. Gut Pathog. 4, 3 (2012).
Wadolkowski, E. A., Laux, D. C. & Cohen, P. S. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of growth in mucus. Infect. Immun. 56, 1030–1035 (1988).
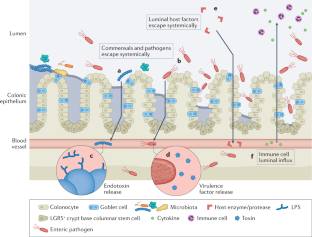
Hausmann, A. et al. Intestinal epithelial NAIP/NLRC4 restricts systemic dissemination of the adapted pathogen Salmonella Typhimurium due to site-specific bacterial PAMP expression. Mucosal Immunol. 13, 530–544 (2020).
Di Domenico, E. G., Cavallo, I., Pontone, M., Toma, L. & Ensoli, F. Biofilm producing Salmonella Typhi: chronic colonization and development of gallbladder cancer. Int. J. Mol. Sci. 18, 1887 (2017).
Johnson, R. et al. Comparison of Salmonella enterica Serovars Typhi and Typhimurium reveals typhoidal serovar-specific responses to bile. Infect. Immun. 86, e00490-17 (2018).
Zha, L., Garrett, S. & Sun, J. Salmonella Infection in chronic inflammation and gastrointestinal cancer. Diseases 7, 28 (2019).
Carter, G. P. et al. Defining the roles of TcdA and TcdB in localized gastrointestinal disease, systemic organ damage, and the host response during Clostridium difficile infections. mBio 6, e00551 (2015).
Mimuro, H. et al. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe 2, 250–263 (2007).
Costa, A. M., Leite, M., Seruca, R. & Figueiredo, C. Adherens junctions as targets of microorganisms: a focus on Helicobacter pylori. FEBS Lett. 587, 259–265 (2013).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Jakobsson, H. E. et al. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS ONE 5, e9836 (2010).
Tourret, J. et al. Immunosuppressive treatment alters secretion of ileal antimicrobial peptides and gut microbiota, and favors subsequent colonization by uropathogenic Escherichia coli. Transplantation 101, 74–82 (2017).
Jones, T. A., Hernandez, D. Z., Wong, Z. C., Wandler, A. M. & Guillemin, K. The bacterial virulence factor CagA induces microbial dysbiosis that contributes to excessive epithelial cell proliferation in the Drosophila gut. PLoS Pathog. 13, e1006631 (2017).
Ferreyra, J. A. et al. Gut microbiota-produced succinate promotes C. difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe 16, 770–777 (2014).
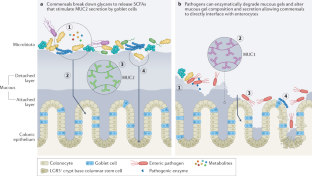
Johansson, M. E. V., Sjövall, H. & Hansson, G. C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361 (2013). A thorough review encompassing the structure and function of mucous within the intestines during health and disease, particularly parasitic and bacterial diseases.
Wheeler, K. M. et al. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat. Microbiol. 4, 2146–2154 (2019).
Furter, M., Sellin, M. E., Hansson, G. C. & Hardt, W. D. Mucus architecture and near-surface swimming affect distinct Salmonella Typhimurium infection patterns along the murine intestinal tract. Cell Rep. 27, 2665–2678.e3 (2019).
Johansson, M. E. V., Larsson, J. M. H. & Hansson, G. C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc. Natl Acad. Sci. USA 108, 4659–4665 (2011).
Johansson, M. E. V. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008).
Li, H. et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat. Commun. 6, 8292 (2015).
Bäckhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A. & Gordon, J. I. Host-bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005).
Engevik, M. A. et al. Human Clostridium difficile infection: altered mucus production and composition. Am. J. Physiol. Gastrointest. Liver Physiol. 308, G510–G524 (2014).
Johansson, M. E. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS ONE 7, e41009 (2012).
Johansson, M. E. V. et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe 18, 582–592 (2015).
Feng, Y. et al. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS ONE 14, e0218384 (2019).
Kim, S. et al. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 13, 1–20 (2021).
Wrzosek, L. et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 11, 61 (2013).
Valeri, M. et al. Pathogenic E. coli exploits SslE mucinase activity to translocate through the mucosal barrier and get access to host cells. PLoS ONE 10, e0117486 (2015).
Luo, Q. et al. Enterotoxigenic Escherichia coli secretes a highly conserved mucin-degrading metalloprotease to effectively engage intestinal epithelial cells. Infect. Immun. 82, 509–521 (2014).
Wilson, K. H. & Perini, F. Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect. Immun. 56, 2610–2614 (1988).
Farquhar, M. G. & Palade, G. E. Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412 (1963).
Drolia, R. & Bhunia, A. K. Crossing the intestinal barrier via Listeria adhesion protein and internalin A. Trends Microbiol. 27, 408–425 (2019).
Drolia, R., Tenguria, S., Durkes, A. C., Turner, J. R. & Bhunia, A. K. Listeria adhesion protein induces intestinal epithelial barrier dysfunction for bacterial translocation. Cell Host Microbe 23, 470–484.e7 (2018).
Fattinger, S. A. et al. Salmonella Typhimurium discreet-invasion of the murine gut absorptive epithelium. PLoS Pathog. 16, e1008503 (2020).
Meza-Segura, M. et al. SepA enhances Shigella invasion of epithelial cells by degrading alpha-1 antitrypsin and producing a neutrophil chemoattractant. mBio 12, e0283321 (2021).
Das, S. et al. Hemochromatosis drives acute lethal intestinal responses to hyperyersiniabactin-producing Yersinia pseudotuberculosis. Proc. Natl Acad. Sci. USA 119, e2110166119 (2022).
Guttman, J. A. & Finlay, B. B. Tight junctions as targets of infectious agents. Biochim. Biophys. Acta 1788, 832–841 (2009). An extensive review highlighting the profound effect pathogen-induced breakdown of tight junctions has on human health and disease...
Comments
Post a Comment